|
| Friday, 17 May 2013, 11:30 HKT/SGT | |
| |  | |
Source: A*STAR | |
|
|
|
|
| The vaccine based on Cytos' Virus-Like Particle technology may open the door to accelerated production of influenza vaccines in Singapore |
Singapore and Schlieren (Zurich), Switzerland, May 17, 2013 - (ACN Newswire) - Singapore's Agency for Science, Technology and Research (A*STAR) and Switzerland's Cytos Biotechnology AG today announced that the first healthy volunteer has been dosed in a Phase 1 clinical trial with their H1N1 influenza vaccine candidate based on Cytos' proprietary bacteriophage Qbeta virus-like particle (VLP) technology. In this first Phase 1 clinical trial, the safety and immunogenicity of this novel vaccine candidate and its potential to protect against H1N1 influenza infection will be evaluated.
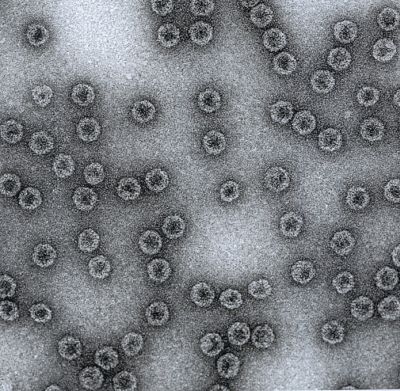 | | Image of Cytos' Virus-Like Particle (VLP) used in Singapore's first H1N1 influenza vaccine |
A*STAR is developing the vaccine candidate under a collaborative research, development and commercialization agreement entered into with Cytos in 2010(1), with the goal of providing the government of Singapore an effective means of combatting influenza epidemics and pandemics. Under the agreement, Cytos retains the worldwide right to develop and commercialize the vaccine candidate globally, while A*STAR subsidiaries will have the right to develop and commercialize the vaccine for Singapore and other ASEAN countries and can earn royalties on worldwide net sales.
Mr Lim Chuan Poh, Chairman of A*STAR and Co-Chair of the Biomedical Sciences Executive Committee in Singapore(2) said, "This is the first time Singapore is attempting to make its own flu vaccine. In the wake of the recent H7N9 bird flu outbreak, it is timely that A*STAR is bringing Singapore's first H1N1 flu vaccine into Phase 1 clinical trial. This different approach of making flu vaccines will better respond to the needs of a flu outbreak. I am pleased that the collaboration with Cytos is making a meaningful contribution to Singapore's pandemic readiness, a critical aspect of our national security. The success of this potential vaccine will be of significant impact not only to the region but also the world."
Christian Itin, PhD, Chairman and Chief Executive Officer of Cytos, commented, "We are very pleased with the fruitful collaboration which has led to the clinical start of this novel influenza vaccine. This is an important milestone for the program and the first clinical program using Cytos' B-cell vaccine platform for a prophylactic vaccine against an infectious disease."
Professor Alex Matter, Chief Executive Officer of D3 (Drug Discovery and Development) and A*STAR's Experimental Therapeutics Centre (ETC) said, "If this VLP-vaccine strategy proves to be effective, it can accelerate the production of vaccines against new emerging strains of flu. This will greatly aid Singapore's preparedness to produce vaccines quickly, safely and economically in the event of a flu epidemic. This could potentially open doors for faster production of vaccines to a range of viral diseases as well."
A*STAR's Experimental Therapeutics Centre (ETC) was the primary driver of the multi-institutional effort culminating in the start of the clinical development program, which involved academic and clinical partners across Singapore, namely A*STAR's Singapore Immunology Network (SIgN), DSO National Laboratories and Duke-NUS Graduate Medical School. Since early 2012, D3, which works hand in hand with Cytos and other local entities, has been leading the development of the H1N1 influenza vaccine project aiming to achieve proof-of-concept in humans. This Phase 1 clinical trial is being conducted at the SingHealth Investigational Medicine Unit and the Changi General Hospital Trials and Research unit in Singapore.
(1) http://bit.ly/19BN2dN
(2) http://www.nrf.gov.sg/nrf/strategic.aspx?id=380
(3) Monto, A.S. (2008). Epidemiology of influenza, Vaccine 26 Suppl 4, D45-48
About Influenza Infection and Vaccination
According to the World Health Organisation, it is estimated that 10 to 20 percent of the world's population are infected during seasonal influenza epidemic, causing three to five million people to be seriously ill, and killing 250,000 to 500,000 each year(3). One of the most effective ways to significantly reduce the incidence of death and serious illness from influenza is through vaccination. In the case of a pandemic, it is vital to be able to produce vaccines quickly and in large quantities to be able to vaccinate the majority of the population prior to the spreading of the pandemic influenza strain. The conventional way to produce influenza vaccine using chicken eggs has limited yields, is time consuming and sometimes constrained due to the toxicity of certain viral strains to birds (e.g. H5N1 bird flu virus). Growing the virus in cell cultures may get around strain toxicity, however the yields are also modest and the production cost are very high.
About the Influenza Vaccine Developed By A*STAR and Cytos
Cytos' proprietary technology used for this vaccine overcomes the short comings of conventional vaccines since all components can be produced recombinantly at high levels in E.coli. The vaccine encompasses on the one hand Cytos' clinically validated Qbeta VLP as an immunological carrier and on the other hand the globular domain of hemagglutinin (HA) which is displayed on the surface of the VLP. In the resulting non-infectious vaccine the influenza antigen derived from the surface protein HA is presented in a highly immunogenic manner to the immune system.
About the Agency for Science, Technology and Research (A*STAR)
The Agency for Science, Technology and Research (A*STAR) is Singapore's lead public sector agency that fosters world-class scientific research and talent to drive economic growth and transform Singapore into a vibrant knowledge-based and innovation driven economy. In line with its mission-oriented mandate, A*STAR spearheads research and development in fields that are essential to growing Singapore's manufacturing sector and catalysing new growth industries. A*STAR supports these economic clusters by providing intellectual, human and industrial capital to its partners in industry. A*STAR oversees 20 biomedical sciences and physical sciences and engineering research entities, located in Biopolis and Fusionopolis as well as their vicinity. These two R&D hubs house a bustling and diverse community of local and international research scientists and engineers from A*STAR's research entities as well as a growing number of corporate laboratories. For more information about A*STAR, please visit www.a-star.edu.sg .
About D3
D3 (Drug Discovery and Development) was established in 2012 to build strong bridges between basic science and clinical research and development by bringing early-stage scientific discoveries to 'proof-of-concept' clinical trials in humans and generating economic benefit through the licensing of clinical stage therapeutics. D3 builds on Singapore's existing drug discovery capabilities and strengthens the local biomedical innovation landscape. It was founded to be a cost-effective and professional development partner able to advance and add value to early-stage projects on a 'shared-risk, shared-reward' basis. D3's primary focus is on developing drugs targeted at oncology indications and infectious diseases but the door is open to other indications if a partner brings the necessary disease knowledge. D3 is jointly funded by A*STAR, the Ministry of Health's National Medical Research Council and the National Research Foundation. For more information about D3, please visit www.d3.a-star.edu.sg .
About Cytos Biotechnology AG
Cytos is a public biopharmaceutical company focused on the development of targeted immuno-therapies. The Company's lead product candidate CYT003 is a first-in-class immune modulator in Phase 2 clinical development as a potential new treatment for allergic asthma. CYT003 acts via a novel, allergen-independent mechanism of action to selectively suppress the body's immune response to allergens, a predominant risk factor for asthma. In a successfully completed Phase 2a study, CYT003 was shown to maintain asthma control and lung function and asthma control in patients with persistent allergic asthma, even as standard inhaled corticosteroid treatment was withdrawn. CYT003 has been shown to be safe in over 450 patients to date. Cytos was founded in 1995 as a spinoff from the Swiss Federal Institute of Technology (ETH) in Zurich. It is located in Schlieren (Zurich), Switzerland. The Company is listed according to the Main Standard on the SIX Swiss Exchange Ltd under the symbol CYTN.
Contact:
Agency for Science, Technology and Research (A*STAR)
Sarah Chang, Ph.D.
Tel: +65 6826 6442
Email: chang_kai_chen@a-star.edu.sg
Cytos Biotechnology AG
Harry Welten
Chief Financial Officer
Tel: +41 44 733 46 46
e-mail: harry.welten@cytos.com
US Investor enquiries
Susan A. Noonan
Tel: +1 (212) 966 3650
e-mail: susan@sanoonan.com
Topic: Research and development
Source: A*STAR
Sectors: Science & Research, BioTech
http://www.acnnewswire.com
From the Asia Corporate News Network
Copyright © 2026 ACN Newswire. All rights reserved. A division of Asia Corporate News Network.
|
|
|

|
|
|
|
| A*STAR |
| June 19, 2025 08:30 HKT/SGT |
|
ULVAC Continues Participation in "Lab-in-Fab" Project to Advance Piezoelectric MEMS Technology, Now Entering a New Phase |
| Dec 6, 2022 14:00 HKT/SGT |
|
Global pharma giants partner Singapore researchers to boost innovation in biologics and vaccines manufacturing |
| June 2, 2022 21:00 HKT/SGT |
|
Boehringer Ingelheim Enters Global Licensing Agreement to Develop and Commercialize Innovative Antibodies from A*STAR for Targeted Cancer Therapies |
| June 2, 2022 21:00 HKT/SGT |
|
Boehringer Ingelheim Enters Global Licensing Agreement to Develop and Commercialize Innovative Antibodies from A*STAR for Targeted Cancer Therapies |
| Sept 30, 2021 16:00 HKT/SGT |
|
A*STAR and Local SME Work with Vaccination Centres to Deploy AVID System for Filling Syringes |
| July 31, 2020 08:00 HKT/SGT |
|
Singapore Cancer Drug ETC-159 Advances Further in Clinical Trials |
| July 24, 2020 17:00 HKT/SGT |
|
MP Biomedicals and A*STAR Co-Develop Rapid Antibody Test Kit for SARS-CoV-2 |
| Oct 22, 2019 04:00 HKT/SGT |
|
Fujitsu, SMU and A*STAR Launch Digital Platform Experimentation Project using Quantum-Inspired Computing and Deep Learning Technology |
| June 28, 2019 08:00 HKT/SGT |
|
Singapore's Drug Development Efforts Given Additional Momentum with National Platforms |
| Apr 5, 2019 18:00 HKT/SGT |
|
Passing of Dr Sydney Brenner, Nobel Laureate, Renowned Pioneer in Molecular Biology, A*Star Senior Fellow |
| More news >> |
 |
|
 |
|