|
| Friday, 9 October 2020, 15:00 HKT/SGT | |
| |  | |
Source: Olympus | |
|
|
|
|
| ~ Setting the foundation for a new era of endoscopy ~ |
HAMBURG & TOKYO, Oct 9, 2020 - (ACN Newswire) - Olympus Corporation today announced the launch of ENDO-AID(*1), a cutting-edge platform powered by artificial intelligence (AI) that includes the endoscopy application ENDO-AID CADe (computer-aided detection) for the colon. This new AI platform enables real-time display of automatically detected suspicious lesions and works in combination with Olympus' recently introduced EVIS X1, its most advanced endoscopy system to date.
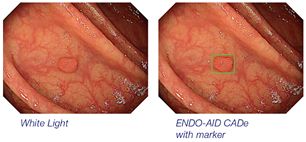 | | ENDO-AID CADe: real-time computer-aided detection for endoscopy |
 | | ENDO-AID |
 | | ENDO-AID with EVIS X1 |
As a global leader in designing and delivering innovative solutions for medical and surgical procedures, Olympus aims to improve the health and quality of life of patients by providing advanced medical technologies. Its latest endoscopy system, EVIS X1, was developed to set new standards for image detection, characterization, staging, and treatment. With the newly introduced endoscopy CAD (computer-aided detection/diagnosis) platform ENDO-AID, Olympus is now enhancing the capabilities of EVIS X1 through AI technology, aiming to elevate the standard of endoscopy around the world - whether for applications to disorders of the esophagus, stomach, colon or other gastrointestinal organs.
ENDO-AID CADe: real-time computer-aided detection for endoscopy
ENDO-AID CADe is an application for computer-aided detection powered by AI which runs on ENDO-AID. It uses a complex algorithm via a neural network developed and trained by Olympus. With this new application, the system's sophisticated machine learning can alert the endoscopist in real time when a suspected colonic lesion (such as a polyp, malignant neoplasm or adenoma) appears on the screen.
ENDO-AID CADe was developed toward the following improvements:
- Improved observational performance in adenoma detection: By providing visual support, ENDO-AID CADe aims to improve the observational performance of the endoscopist's adenoma detection.
- Support of the colonoscopy screening process: The system provides visual support during screening, allowing the endoscopist to focus on any abnormalities indicated by the software regardless of the experience level of the endoscopist.
- Efficient endoscopy operation: Due to the simple and intuitive display of lesions, ENDO-AID CADe has the potential to make endoscopy easier and more efficient for the endoscopist by reducing the need for excessive eye movements.
A first step towards the future of endoscopic diagnosis and therapy
With ENDO-AID, the latest feature of the EVIS X1 endoscopy system, Olympus has created the basis and infrastructure for the installation of future applications supported by AI. "At Olympus we are committed to innovation and driving our research and development with passion," says Frank Drewalowski, Head of Endoscopic Solutions Division, Olympus Corporation. "Especially in AI, we recognize the power of elevating endoscopic imaging to uncharted levels. Considering ENDO-AID as a first step, we are planning additional AI-powered applications for image detection and characterization - not only for colonoscopy."
Prevention of colorectal cancer remains our focus
There is a positive impact of an increase in adenoma detection rate (ADR) on the prevention of colorectal cancer (CRC)(*2). Supporting the identification of lesions, ENDO-AID CADe is designed to increase ADR(*3). We are aiming to increase the quality of CRC screening and its preventive efficacy against CRC. The prevention of CRC is a core element of Olympus' endeavors in medical endoscopy and beyond.
"With the launch of ENDO-AID, we are not only providing endoscopists across the world with an additional innovative tool," says Takaharu Yamada, Vice President, GI Endoscopy Business Leader, Endoscopic Solutions Division. "We are also preparing for the future and following our vision of putting CRC in the history books."
Official launch at United European Gastroenterology Week (UEGW)
During the virtual UEGW from October 11 to 13, ENDO-AID will be presented to the public for the first time. ENDO-AID is initially and commercially being launched in Europe beginning in November, followed shortly afterwards by some Middle Eastern, African and Asian-Pacific countries. Japan, the Americas and China markets will follow at a later time after complying with laws and regulations in each region. EVIS X1 and ENDO-AID are manufactured by Olympus Medical Systems Corporation. The company and product names specified in this release are the trademarks or registered trademarks of Olympus.
(*1) Product Name: Endoscopy CAD system
(*2) Corley, D.A.; Jensen, C.D.; Marks, A.R.; et al. Adenoma Detection Rate and Risk of Colorectal Cancer and Death. N Engl J Med. 2014; 370: 1298-1306. Available at:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4036494/. Accessed in October 2020
(*3) Compared to WLI (white light imaging) without CADe
About Olympus
Olympus is passionate about the solutions it creates for the medical, life sciences, and industrial equipment industries, as well as cameras and audio products. For more than 100 years, Olympus has focused on making people's lives healthier, safer, and more fulfilling by helping detect, prevent, and treat disease, furthering scientific research, ensuring public safety, and capturing images of the world.
Olympus Endoscopic Solutions uses innovative capabilities in medical technology, therapeutic intervention, and precision manufacturing to help healthcare professionals deliver diagnostic, therapeutic, and minimally invasive procedures to improve clinical outcomes, reduce overall costs, and enhance the quality of life for patients. Starting with the world's first gastrocamera in 1950, Olympus' endoscopic portfolio has grown to include endoscopes, laparoscopes, and video imaging systems, as well as systems integration solutions and medical services.
Source: https://www.olympus-global.com/news/2020/contents/nr01857/nr01857_00001.pdf
For questions or additional information, please contact:
Europe, Middle East and Africa
Matthias Gengenbach
+49 15142369420
matthias.gengenbach@olympus-europa.com
Japan
Yuka Horimoto
+81 90 2490 1071
yuka_horimoto@ot.olympus.co.jp
Asia Pacific
Oliver Clarke
+61 3 9271 5508
oliver.clarke@olympus.com.au
Topic: Press release summary
Source: Olympus
Sectors: BioTech, Healthcare & Pharm, Artificial Intel [AI]
http://www.acnnewswire.com
From the Asia Corporate News Network
Copyright © 2026 ACN Newswire. All rights reserved. A division of Asia Corporate News Network.
|
|
|

|
|
|
|
| Olympus |
| Feb 3, 2026 09:00 HKT/SGT |
|
EAGLE Trial Shows Olympus(R) CADDIE(TM) AI Solution Aids in the Detection of High-Risk and Hard-to-Detect Colorectal Lesions |
| Dec 15, 2025 07:30 HKT/SGT |
|
Olympus Triples Venture Capital Fund Investment to Strengthen MedTech Leadership |
| Nov 7, 2025 14:30 HKT/SGT |
|
Olympus Unveils Corporate Strategy |
| Oct 29, 2025 10:00 HKT/SGT |
|
Olympus Introduces NBI+TXI(TM) Observation Mode to the EVIS X1(TM) Endoscopy System CV-1500 Video System Center |
| Oct 21, 2025 22:00 HKT/SGT |
|
Olympus Launches New Surgical Energy Device for Hemostatic Cutting and Vessel Sealing |
| Oct 21, 2025 10:00 HKT/SGT |
|
Olympus: "Feasibility study on the development of Japanese digitalized endoscopy infection control systems in India" Selected for METI's FY2024 Supplementary "Global South Future-Oriented Co-Creation Subsidy Program (Small-Scale Demonstration / Feasibility Study Project)" |
| Oct 6, 2025 14:00 HKT/SGT |
|
Olympus Launches SecureFlex, a Single-use Fine Needle Biopsy Device |
| Sept 30, 2025 21:00 HKT/SGT |
|
Olympus Launches OLYSENSE CAD/AI in the US and Europe |
| Sept 30, 2025 12:00 HKT/SGT |
|
Olympus Commits to Advancing Sustainable Packaging Enabled by DuPont(TM) Tyvek(R) with Renewable Attribution |
| July 25, 2025 15:00 HKT/SGT |
|
Olympus Enters Strategic Partnership to Develop Endoluminal Gastrointestinal Robotics |
| More news >> |
 |
|
 |
|