Tokio Marine & Nichido Fire Insurance Co., Ltd. and Eisai Co., Ltd. announced that they have co-developed “Dementia Care Support Insurance” to financially support early detection and early treatment for dementia as a part of their business alliance for the realization of a Dementia Inclusive Society.
Background
Tokio Marine Nichido and Eisai concluded a business alliance agreement for the realization of a Dementia Inclusive Society in August 2019, and the two companies have promoted initiatives to achieve this purpose, including the provision since April 2021 of “NouKNOW” (1), a digital tool for self-assessment of cognitive function developed by Eisai, as an ancillary service for “Long-term care indemnity (installment payment)”, sold by Tokio Marine Nichido.
Dementia was thought to be difficult to treat, but with the approval of a new drug for Alzheimer's disease, which accounts for 60% of dementia cases, and the hope of slowing disease progression by starting treatment in the early stages of disease, the preparation for early detection and early treatment is becoming increasingly important.
Amid such circumstances, the two companies co-developed a new insurance product, “Dementia Care Support Insurance” to financially support early detection and early treatment of the disease, with the expertise Tokio Marine Nichido has developed through dealing with insurance products and related services and Eisai’s extensive experience in the field of dementia.
Overview of (Industry’s First(2)) “Dementia Care Support Insurance”
a. Details of Coverage/Service
The new treatment is indicated for patients with mild cognitive impairment and mild cognitive impairment due to Alzheimer's disease, but determination of eligibility requires medical tests, including a PET scan, to confirm amyloid-β pathology. Since these tests and treatments require a certain level of out-of-pocket expenses, the following coverage will be provided for financial support.
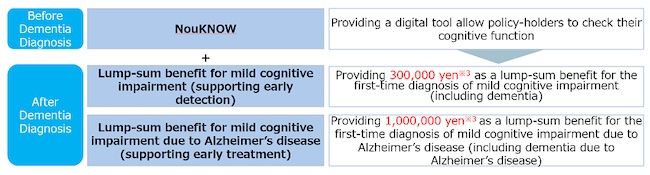
- Lump-sum benefit for mild cognitive impairment (Support for early detection)
In the event of the first diagnosis of mild cognitive impairment (including dementia), a lump sum benefit for the purpose of covering costs for amyloid PET tests, etc. is provided.
- Lump-sum benefit for mild cognitive impairment due to Alzheimer’s disease (Support for early stage treatment)
In the event of the first diagnosis of mild cognitive impairment due to Alzheimer’s disease (including dementia due to Alzheimer’s), a lump-sum benefit for the purpose of covering medical costs for treatment with the new drug is provided.
Additionally, the policy provides the opportunity to use “NouKNOW” as an ancillary service, ensuring the support of early detection and early treatment by identifying cognitive decline.
b. Process Upon Signing The Policy
Potential policy holders need to complete a “NouKNOW" test to purchase the policy, along with completing a Statement of Health form.
c. Insurance Premium
Monthly 1,370 yen (in the case of a male aged 50 to 54 years receiving a 300,000 yen lump-sum benefit for mild cognitive impairment, and 1 million yen for mild cognitive impairment due to Alzheimer's disease). *May vary depending on terms of contract
d. Type of Policy
A group insurance in which a large-scale entity, such as a company, purchases the policy and its members voluntarily purchase the coverage through the entity.
3. Future Perspectives
Tokio Marine Nichido and Eisai will further promote efforts to resolve various social challenges by expanding our network through collaboration with various companies and organizations, to realize a Dementia Inclusive Society.
(1) A tool for checking brain reaction speed, attention, visual learning and memory through four types of simple tests with images of playing cards on a PC, tablet, or smartphone. (Not a medical device)
(2) The first coverage in the non-life insurance industry focusing on mild cognitive impairment due to Alzheimer’s disease and dementia due to Alzheimer’s disease (according to a survey conducted by Tokio Marine Nichido)
(3) May vary depending on terms of contract
Media Contacts:
Tokio Marine & Nichido Fire Insurance Co., Ltd.
Public Relations:
TEL:+81-(0)3-6704-4268
Eisai Co., Ltd.
Public Relations:
TEL:+81-(0)3-3817-5120
Topic: Press release summary
Source: Eisai
Sectors: BioTech
http://www.acnnewswire.com
From the Asia Corporate News Network
Copyright © 2026 ACN Newswire. All rights reserved. A division of Asia Corporate News Network.
|