Eisai Co., Ltd. announced today that the company has achieved the pre-set performance indicators in FY2023 for the dementia examination project in Bunkyo City, Tokyo, which the company has been commissioned to conduct in order to raise awareness of dementia and promote early detection, and will continue the commission for this project in FY2024.
This project is a dementia examination program targeting approximately 12,300 Bunkyo City residents between the ages of 55 and 75, at a milestone of every 5 years. Brain health is checked using “NouKNOW” (pronounced “NOH-NOH”), a digital tool for self-assessment of cognitive function, and based on the assessment results as well as a medical interview, physicians will provide medical advice or referrals to medical institutions. The project also introduces support available from visiting nurses, and encourages participation in lifestyle improvement programs. Since early detection and response to dementia is important, Tokyo is recommending that the target age group for dementia examination be expanded from the current age of 70 and over to 50 and over. This project, which targets 55-year-olds, is one of the pioneering examples of local government efforts toward the early detection of dementia.
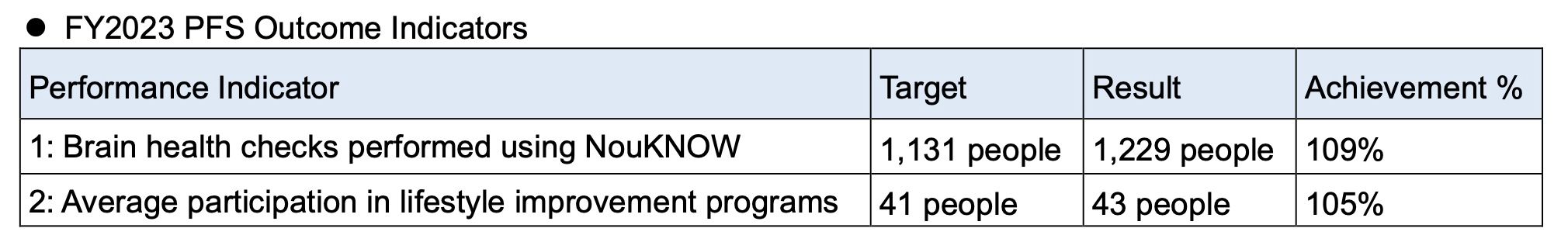
The dementia examination project has been implemented since FY2021 as a priority policy of Bunkyo City, and Eisai has been commissioned for the project since it’s launch, providing NouKNOW and operational support for their public examinations. In FY2022, a Pay For Success (PFS) contract was introduced and targets were established for the outcome indicators of number of NouKNOW checks performed and number of participants in lifestyle improvement programs. Eisai achieved both outcome indicators in FY2022 and FY2023 through measures such as holding NouKNOW trial sessions and providing opportunities to experience "Brepacise®," an exercise program developed by Eisai.
In FY2023, 245 people participated in on-site examinations, of which approximately 12% led to recommendations to see a medical institution. In a survey conducted after the examination, 52% of the 122 respondents said that they had developed a more positive outlook on dementia, and 68% said that they had become more interested in the brain and health.
Eisai has concluded regional cooperation agreements with local governments, medical associations, and other organizations throughout Japan, providing opportunities for brain health assessments tailored to local issues and conducting initiatives to establish a pathway to subsequent medical care and support. In FY2023, these initiatives were promoted in 60 local governments. Moving forward, Eisai will continue to contribute to the creation of a community where local residents are aware of and check their brain health from the stage where they are in a healthy condition, and where early detection, diagnosis, and preparation for dementia are possible, aiming to realize a Dementia-Inclusive Society where people with dementia can live their lives how they would like.
About NouKNOW
NouKNOW (non-medical device) is a tool that uses a simple card test using a PC, tablet or smartphone device to perform tests evaluating psychomotor function, attention, learning and memory, and working memory. This digital tool allows users to self-assess independently and in a short time frame (approx.15 minutes), enabling regular assessments in instances such as daily life and health checkups. It has been adopted by a number of medical and research institutions, local governments, corporations and universities.
For additional information, please visit https://nouknow.jp/. (Japanese only)
(List of partners: https://nouknow.jp/partner/)
About Pay For Success (PFS)
Pay For Success (PFS) is a new method of public-private partnership to set outcome indicators in accordance with the administrative issues to be solved by the projects that local public entities, etc. contract out to private sectors for its implementation, and the amount paid by the local public entity, etc. to the private sector for outsourcing a project is linked to improvement of the relevant outcome indicators as evaluated by a third party. PFS is a policy promoted by the Cabinet Office of Japan, which is expected to improve the quality of public services and reduce expenditures.
About Brepacise
Brepacise is a dual-task exercise program that stimulates the body and brain by moving the arms and legs to music while also incorporating intellectual challenges. Eisai developed this product with the review of a physician as an easy and fun introductory exercise for those concerned about their brain health. It is being used in health classes and online events run by local governments.
For additional information, please visit the dementia information site “Sodan e-65” operated by Theoria technologies Inc., a member of Eisai Group. (https://soudan-e65.com/maintenance/brain_exercise/, Japanese only)
Media Inquiries:
Public Relations Department
Eisai Co., Ltd.
+81-(0)3-3817-5120
Topic: Press release summary
Source: Eisai
Sectors: BioTech
http://www.acnnewswire.com
From the Asia Corporate News Network
Copyright © 2026 ACN Newswire. All rights reserved. A division of Asia Corporate News Network.
|