TOKYO, Jan 7, 2025 - (JCN Newswire) - A collaborative research group led by Professor Kenjiro Ono of the Institute of Medical, Pharmaceutical and Health Sciences, Kanazawa University, and Eisai Co., Ltd., used a newly developed measurement system for lecanemab-associated amyloid-β protofibrils (Lec-PF) to demonstrate that the concentration of Lec-PF in human cerebrospinal fluid is elevated in patients withAlzheimer's disease at all stages, from mild cognitive impairment to mild and severe dementia, compared with non-Alzheimer's disease controls. Furthermore, this research identified that the concentration of Lec-PF in cerebrospinal fluid is significantly correlated with biomarkers reflecting neurodegeneration.
Lecanemab (*1) is a unique dual acting antibody that selectivity binds to amyloid-β protofibril (PF *2) in addition to removing plaque, and is a treatment for patients with mild cognitive impairment and mild dementia due to Alzheimer’s disease (AD *3) (collectively referred to as early AD). The results of this study suggest that PF which is captured by lecanemab (Lec-PF), is present in the cerebrospinal fluid of patients with Alzheimer’s disease, and that Lec-PF is a highly toxic pathological protein that causes neurodegeneration. This research has revealed part of the mechanism by which lecanemab demonstrates its effect of slowing the progression of Alzheimer’s disease.
Based on these findings, it may be possible in the future to measure Lec-PF concentration in cerebrospinal fluid before and afterlecanemab treatment to assess the treatments efficacy. In addition, because the concentration of Lec-PF in cerebrospinal fluid correlates well with neurodegenerative markers such as total tau (*4) and neurogranin (*5), it is expected that these findings could also be useful in predicting the prognosis of Alzheimer’s disease patients.
This research was published in the online version of the international academic journal Annals of Neurology at 18:30 on January 6, 2025 (US Eastern Standard Time).
Research Background and Objectives
The amyloid cascade hypothesis is a leading theory regarding the cause of AD, which states that amyloid beta (Aβ) (*6) abnormally aggregates outside of neuron, followed by the formation of tau neurofibrillary tangles, which causes damage to neuron and leads to the onset of the disease. PF is more neurotoxic than amyloid fibrils and plaque, which are the final stages of abnormal aggregated Aβ, and it is believed that PF may cause neurodegeneration (reference: Amin L and Harris DA. 2021). Lecanemab is an anti-PF antibody that has been used in medical practice since 2023 as a treatment
for early AD. However, there have been no previous reports of measuring PF concentrations in human biological samples, and the profiles of PF, which is the therapeutic target of lecanemab, across the full AD continuum have not yet been understood.
In this study, the research group used a newly developed, highly sensitive Lec-PF measurement system to measure the concentration of Lec-PF in human cerebrospinal fluid (CSF) in subjects with preclinical AD (*7), mild cognitive impairment due to AD, and AD dementia, as well as patients with SNAP (suspected non-AD pathophysiology) (*8) and people with normal cognitive function, and analyzed the association between Lec-PF and Aβ40, Aβ42, phosphorylated tau (p-tau) 181 (*9), p-tau 217, total tau, and neurogranin.
Summary of Research Results
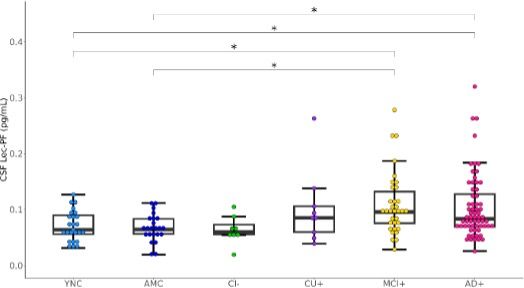
The Lec-PF CSF concentration was significantly higher in patients with mild cognitive impairment or dementia due to AD (MCI+,AD+ groups in Figure 1) than in those with normal cognitive function (YNC and AMC groups in Figure 1).
Correlation analysis was conducted between the concentration of Lec-PF in cerebrospinal fluid and other cerebrospinal fluid markers (Aβ42, Aβ42/40 ratio, p-tau 181, p-tau 217, total-tau, neurogranin).
Future Developments
Measuring the Lec-PF concentration in CSF before and after lecanemab treatment may be useful in assessing the efficacy of lecanemab treatment. In addition, because the concentration of Lec-PF in CSF correlates well with neurodegenerative markers, it may also be useful for predicting the prognosis of AD patients.
This research was supported by the Japan Agency for Medical Research and Development (AMED) Research and Development Grants for Dementia (Grant No. 22dk0207053), Grants-in-Aid for Transformative Research (Grant No. 23H03850), and Grants-in-Aid for Scientific Research (Grant Nos. JP19k07965, JP22k07514).
Fig. 1 Cerebrospinal fluid lecanemab-related amyloid protofibril (CSF Lec-PF) concentrations according to clinical diagnosis and amyloid positivity/negativity
Description of Figure 1
Boxes in boxplots indicate interquartile ranges andhorizontal lines indicate medians.
Abbreviations for each group: YNC (young normal control amyloid-negative), AMC (age-matched control amyloid-negative), CI- (cognitively impaired amyloid-negative), CU+ (cognitively unimpaired amyloid-positive), MCI+ (mild cognitive impairment amyloid-positive), AD+ (AD dementia amyloid- positive), CSF (cerebrospinal fluid)
*p < 0.05
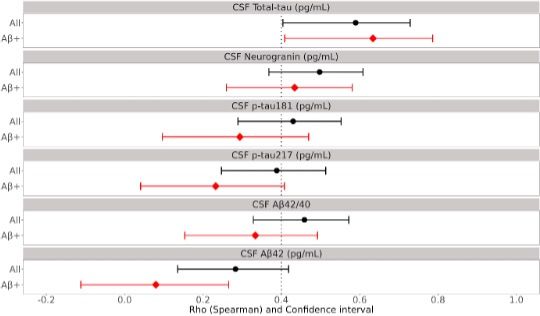
Figure 2 Correlation between cerebrospinal fluid markers and cerebrospinal fluid lecanemab- related amyloid protofibrils (CSF Lec-PF)
Description of Figure 2
The black line and the red line indicate the 95% confidence interval (CI) of the Spearman correlation coefficient between CSFLec-PF and each cerebrospinal fluid marker in the entire subject group and in the amyloid-positive subject group, respectively. The black and red dots indicate the median value of the Spearman correlation coefficients. A large discrepancy between the black and red lines reflects the significant impact of the status of being either Aβ positive or negative.
In this analysis, in all subjects (black line), the correlation coefficients between CSF Lec-PF and all measured biomarkers, namely Aβ42, Aβ42/40 ratio, p-tau 181, p-tau 217, total-tau, and neurogranin, were all 0.2 or higher, indicating significant correlations between CSF Lec-PF and these biomarkers.
In the amyloid-positive group (red line), the Spearman correlation coefficients were 0.634 (CI: 0.409- 0.786) between CSF Lec-PF and CSF total-tau, and 0.434 (CI: 0.260-0.581) between CSF Lec-PF and CSF neurogranin, respectively, indicating a strong correlation between CSF Lec-PF and the two biomarkers. On the other hand, the correlations between CSF Lec-PF and the brain Aβ accumulation biomarkers, CSF Aβ42 and the CSF Aβ42/40 ratio, were both relatively low. This indicates that the amount of CSF Lec-PF is more strongly associated with neurodegeneration than the accumulation of Aβ in the brain.
Published Papers
Journal name: Annals of Neurology
Paper title: Lecanemab-associated amyloid-β protofibril in cerebrospinal fluid correlates with biomarkers of neurodegeneration in Alzheimer’s disease (Cerebrospinal fluid (CSF) lecanemab-associated amyloid protofibril concentrations correlate well with neurodegenerative biomarkers in AD)
Authors: Moeko Noguchi-Shinohara, Kazuyoshi Shuta, Hidetomo Murakami, Yukiko Mori, Junji Komatsu, Chizuru Kobayashi, Steven Hersch, Kanta Horie, Kenjiro Ono (Moeko Shinohara, Kazuyoshi Shuta, Hidetomo Murakami, Yukiko Mori, Junji Komatsu, Chizuru Kobayashi, Steven Hersch, Kanta Horie, Kenjiro Ono)
Date of publication: January 6, 2025 (US Eastern Standard Time). DOI: 10.1002/ANA.27175
Terminology
(1) Lecanemab
Lecanemab is a treatment for early Alzheimer’s disease (AD) and is an antibody that selectively binds to amyloid protofibrils, which are considered to be particularly neurotoxic among amyloid beta aggregates. In September 2023, lecanemab was approved in Japan as a treatment for slowing progression of mild cognitive impairment and mild dementia due to AD.
(2) Protofibrils
Protofibrils are large Aβ aggregated soluble species of 75-5000 kDa, and are believed to contribute to the brain injury that occurs with AD and are considered to be the most toxic form of Aβ, having a primary role in the cognitive decline associated with this progressive, debilitating condition.
(3) Alzheimer’s disease
AD is a progressive brain disorder that gradually impairs memory and cognitive ability, eventually leading to a condition knownas dementia that interferes with daily life. In the brains of patients with AD, changes such as structures called senile plaques that form due to the accumulation of a substance called amyloid-β, abnormal tangles of neurofibrillary changes (neurofibrillarytangles caused by the abnormal phosphorylation of tau protein), and loss of neurons are observed. These changes progress over a long period of time.
(4) Total Tau
Tau protein is a type of microtubule-associated protein that is found in large amounts in neurons and glial cells in the central and peripheral nervous systems. In neurodegenerative diseases, including AD, the concentration of total tau in CSF increases as the disease progresses. For this reason, total tau is used as a marker of neurodegeneration.
(5) Neurogranin
Neurogranin is a neuron-specific postsynaptic protein that is found in large amounts in areas such as the cerebral cortex andhippocampus. Neurogranin in CSF is used as a marker for neurodegeneration.
(6) Amyloid beta (Aβ)
Amyloid-β (Aβ) includes peptides generated by the cleavage of amyloid precursor protein (APP) by β- secretase and γ-secretase, such as Aβ40, which ends at position 40 of the C-terminus, and Aβ42, which ends at position 42 of the C-terminus Aβ42 inparticular has a high aggregating tendency, and in AD, there is a decrease in Aβ42 concentration in CSF and an increase in the Aβ40/42 ratio, which is known to show a strong correlation with the amount of amyloid accumulation measured by amyloid PET testing.
(7) Preclinical Alzheimer’s disease
A stage of AD before cognitive impairment has occurred.
(8) SNAP (suspected non-Alzheimer’s disease pathophysiology)
SNAP is an abbreviation for suspected non-AD pathophysiology. The term refers to a condition that involves neurodegeneration similar to that seen in AD, despite normal results of amyloid PET scans, CSF Aβ40/42 ratio, and CSF Aβ42. In this study, the CI- group (cognitively impaired amyloid-negative group) in Figure 1 corresponds to patients with SNAP.
(9) Phosphorylated tau
The accumulation of phosphorylated tau protein is considered to be a cause of neurofibrillary tangles, which are pathological traits of AD. Many phosphorylation sites are known in tau protein, among which tau protein phosphorylated at threonine residue 181 (p-tau 181) and tau protein phosphorylated at threonine residue 217 (p-tau 217) are frequently seen in AD. For this reason, p-tau 181 and p-tau 217 in CSF are used as biomarkers for AD.
Reference
Amin L, Harris DA. Aβ receptors specifically recognize molecular features displayed by fibril ends and neurotoxic oligomers. Nat Commun. 2021;12:3451. DOI:10.1038/s41467-021-23507-z
Contact for Inquiries
Regarding research content Kanazawa University Professor Kenjiro Ono
TEL: +81-(0)76-265-2292
Email: onoken@med.kanazawa-u.ac.jp
Associate Professor Moeko Shinohara
TEL: +81-(0)76-265-2292
Email: m-nohara@med.kanazawa-u.ac.jp
Eisai Co., Ltd.
Kanta Horie
E-mail: kanta_horie@eisai.com
Public Relations Officer
General Affairs Section, General Affairs Division, Medical, Pharmaceutical and Health Sciences Administration Department, Kanazawa University
Rina Yamada
TEL: +81-(0)76-265-2109
E-mail: t-isomu@adm.kanazawa-u.ac.jp
Eisai Co., Ltd.
PR Department TEL: +81-(0)3-3817-5120
Topic: Press release summary
Source: Eisai
Sectors: BioTech
http://www.acnnewswire.com
From the Asia Corporate News Network
Copyright © 2026 ACN Newswire. All rights reserved. A division of Asia Corporate News Network.
|