|
|
|
|
|
HONG KONG, Feb 17, 2022 - (ACN Newswire) - SinoMab BioScience Limited ("SinoMab" or the "Company", together with its subsidiaries, the "Group", stock code: 3681.HK), a Hong Kong-based biopharmaceutical company dedicated to the research, development, manufacturing and commercialization of therapeutics for the treatment of immunological diseases, is pleased to announce that, on 11 February 2022 (EST local time), an Investigational New Drug application ("IND"), for the Company's First-in-Class (FIC) asthma therapeutic product SM17 (Humanized anti-IL17RB monoclonal antibody for injection) has been submitted and accepted by the U.S. Food and Drug Administration ("FDA"). The Company plans to initiate the First-In-Human study in the U.S. in first quarter of 2022, once IND is approved by FDA.
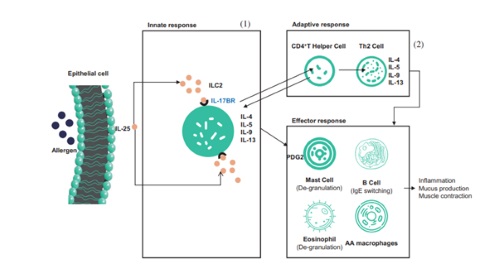 | | SM17's mechanism of action |
SM17 is the world's first monoclonal antibodies targeting IL17BR co-developed by SinoMab and LifeArc (a medical research charity based in the United Kingdom). SM17 has a wide range of indications, including indications with large market volumes such as asthma and diseases with high mortality rates such as idiopathic pulmonary fibrosis. Comparing to other products on the market, SM17 enjoys differentiation advantages. With the preclinical data and unique mechanism of action of SM17, Company believes that SM17 potentially has a broader and more beneficial effect on asthma treatment than other approved biologics.
In the global market, the number of asthma patients is gradually increasing and is expected to reach 247.5 million by 2023 and further increase to 267.7 million by 2030. The number of asthma patients in the PRC is increasing at a greater pace than the global rate and is forecasted to reach 25.6 million by 2023 and further increase to 27.8 million by 2030. In terms of market size, the global asthma market is projected to reach US$25.1 billion by 2023 and US$34.6 billion by 2030. However, the asthma market in the PRC is expected to reach RMB36.4 billion by 2023 and RMB65.0 billion by 2030. In terms of treatment options, traditional asthma treatment is based on inhaled corticosteroid, but they are prone to serious adverse effects, especially in adolescents. Drug resistance can also develop if used for a long time. The introduction of SM17 is expected to provide a better treatment option in terms of the balance of efficacy and safety.
Dr. Shui On LEUNG, Chairman, Executive Director and Chief Executive Officer of SinoMab said that: "following the acceptance of the IND application for SN1011 for the treatment of multiple sclerosis by the NMPA, the acceptance of the SM17 IND application by the FDA fully demonstrates the efficient execution of the Company's new drug R&D program. There is still an unmet medical need for additional effective therapies, particularly for patients who do not respond to current treatments. We are therefore confident in the enormous prospects of SM17's clinical development. Our core products, including SM03, SN1011 and SM17, is making progress on the clinical R&D smoothly, driving the Company moving steadily towards commercialization. In the future, we will accelerate our projects implementation to bring benefits to patients and create value for shareholders through innovation."
About SM17
SM17 is known to be the world's first humanized, IgG4-k monoclonal antibody for new drug development, which targets IL-17RB. And IL-17RB is a type-I single transmembrane glycoprotein belonging to IL-17 receptor family. The binding of SM17 to IL-17RB could suppress Th2 immune responses induced by a category of cytokines called "alarmin", which has shown to be implicated in the pathogenesis of allergic disease and airway viral responses. Alternative approach targeting upstream mediators of the Th2 inflammatory cascade, such as "alarmins", is expected to have a broader effect on airway inflammation and to provide more effective asthma control than currently available therapies, and products with similar mechanism of action as SM17 have been approved by FDA.
About SinoMab BioScience Limited
SinoMab BioScience Limited (stock code: 3681.HK) is dedicated to the research, development, manufacturing and commercialization of therapeutics for the treatment of immunological diseases. The Company's flagship product SM03 is a potential global first-in-target mAb against CD22 for the treatment of rheumatoid arthritis (RA) and is currently in Phase III clinical trial for rheumatoid arthritis in China, which has been recognized as one of the significant special projects of Significant New Drugs Development of the Twelfth Five-Year Plan Period and the Thirteenth Five-Year Plan Period. In addition, the Company possesses other potential first-in-target and first-in-class drug candidates, some of which are already in clinical stage, with their indications covering rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), pemphigus vulgaris (PV), non-Hodgkin's lymphoma (NHL), asthma, and other diseases with major unmet clinical needs.
Topic: Press release summary
Source: SinoMab BioScience Limited
Sectors: BioTech, Healthcare & Pharm
http://www.acnnewswire.com
From the Asia Corporate News Network
Copyright © 2026 ACN Newswire. All rights reserved. A division of Asia Corporate News Network.
|
|
|

|
|
|
|
| SinoMab BioScience Limited |
| Apr 9, 2025 14:49 HKT/SGT |
|
SinoMab BioScience Limited Breakthrough Phase 1b POC Results for SM17 in Atopic Dermatitis |
| Apr 16, 2024 17:35 HKT/SGT |
|
SinoMab BioScience preclinical results of SM17 on atopic dermatitis (AD) published on International Scientific Journal Allergy |
| Sept 12, 2023 09:48 HKT/SGT |
|
SinoMab's IND Application of SM17 has once again Received Approval from NAMP |
| Aug 14, 2023 21:10 HKT/SGT |
|
SinoMab Announces that IND Application of SM17 for the Treatment of Asthma was Approved by NAMP |
| June 12, 2023 20:43 HKT/SGT |
|
SinoMab Submits another IND Application for SM17 for the Treatment of Atopic Dermatitis, Which was Accepted by NMPA CDE |
| May 22, 2023 10:20 HKT/SGT |
|
SinoMab Announces IND Application of SM17 for the Treatment of Asthma was Accepted by NMPA CDE |
| Dec 19, 2022 14:55 HKT/SGT |
|
SinoMab Awarded the "Most Valuable Pharmaceutical and Medical Company" in the Selection of the "7th Hong Kong Golden Stocks Awards" |
| Nov 4, 2022 17:47 HKT/SGT |
|
SinoMab Announces Appointment of Mr. Shanchun WANG as the President (China) of the Company |
| Aug 24, 2022 08:05 HKT/SGT |
|
Sinomab Announces IND Application of SN1011 for the Treatment of Neuromyelitis Optica Spectrum Disorder Approved by NMPA |
| July 8, 2022 19:03 HKT/SGT |
|
SinoMab Received the Highest Subsidy from HKSTP |
| More news >> |
 |
|
 |
|